Abstract
Introduction Treatment of relapsed/refractory (R/R) Hodgkin lymphoma (HL) following failure of brentuximab vedotin (BV) and PD-1 blockade is a major unmet need. Resistance to BV is associated with multidrug resistance gene 1 (MDR1) overexpression. There are pre-clinical data showing that MDR1 inhibitors such as cyclosporine (CsA) or verapamil (VRP) are synergistic with BV in BV-resistant HL cell lines. Preliminary results of a phase I study evaluating the safety and efficacy of combination of BV with CsA and VRP in R/R HL were previously reported. Here we report the final results of the completed study.
Methods This is a single center, open-label phase I investigator-initiated study. Patients (pts) with HL whose disease had relapsed or was refractory to ≥1 prior lines of therapy were enrolled. The dose finding portion followed a 3+3 design with 4 planned dose levels (DL). BV was given at 1.2 mg/kg (DL1) or 1.8 mg/kg (DL2-4) intravenously every 3 weeks. Planned dose of CsA was 5 mg/kg (DL1-2) or 7.5 mg/kg (DL3-4) orally twice daily on days 1-5. Planned dose of VRP was 120 mg orally four times daily on days 1-5 (DL4). Each cycle was 3 weeks. Once the maximum tolerated dose (MTD) was determined, BV-refractory pts were enrolled in an expansion cohort at the MTD. Primary objective was to determine the MTD and safety of the combination. Secondary objectives were overall response rate (ORR), complete response (CR) rate, response duration (DOR), overall survival (OS), and progression-free survival (PFS).
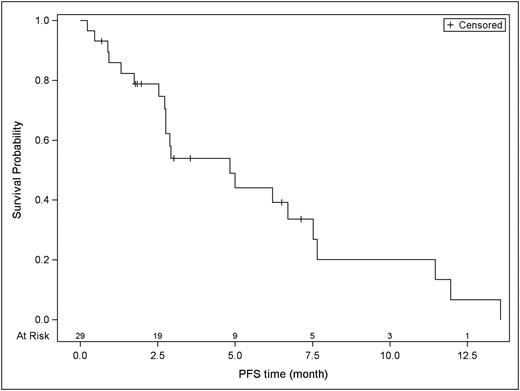
Results Twenty-nine pts were enrolled onto the study, 14 in the dose-finding portion and 15 in the dose expansion BV-refractory cohort. With respect to baseline characteristics, 38% of patients were female, 69% had advanced stage disease, 45% had extranodal disease, and 24% had B symptoms. Median age was 36 (20-69) and pts had a median of 5 (3-12) prior lines of therapy. All had prior BV (only 2 BV-sensitive) and 27 had prior PD-1 blockade. Four pts were treated on DL1, 22 on DL2, and 3 on DL3. MTD was DL2. DLTs observed were: DL1 10-day CsA (n=1) grade (Gr) 3 hyperbilirubinemia, abdominal pain, and hypertension, DL2 (n=1) Gr3 abdominal pain and Gr3-4 neutropenia, DL3 (n=2) Gr3 bone pain/constipation/Gr4 lymphopenia (n=1) and Gr3 hyperglycemia (n=1). Median duration of treatment was 3 (1-16) cycles. Most frequent adverse events (AEs) were nausea (90%), hypertension (90%), anemia (86%), fatigue (76%), neutropenia (76%), and leukopenia (76%). All pts had Gr3+ AEs with most frequent being neutropenia (62%). Reasons for treatment discontinuation included death (n=4), disease progression (n=10), toxicity (n=2), patient refusal (n=8), loss to follow-up (n=2), MD decision (n=1), proceeding to HCT (n=1), and study completion (n=1). Treatment-related death occurred in 3 pts (pneumonitis at DL1, respiratory failure and hypotension at DL2). The ORR/CR was 62%/24%. The median DOR and PFS was 5 months and median OS was not reached.
Discussion The combination of BV and CsA was effective in BV-refractory R/R HL but was also associated with toxicity, including treatment-related deaths that led to early termination of the clinical trial.
Disclosures
Mei:EUSA: Honoraria; Celgene: Research Funding; Morphosys: Research Funding, Speakers Bureau; CTI: Honoraria; Novartis: Consultancy; Incyte: Research Funding; Beigene: Research Funding. Rosen:January Therapeutics: Current holder of stock options in a privately-held company. Herrera:Bristol-Myers Squibb: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Gilead: Research Funding; Takeda: Consultancy; Tubulis: Consultancy; AstraZeneca: Consultancy, Research Funding; Regeneron: Consultancy; Seattle Genetics: Consultancy, Research Funding; KiTE Pharma: Research Funding; Adicet Bio: Consultancy; Caribou: Consultancy; Pfizer: Consultancy; Genmab: Consultancy; Karyopharm: Consultancy; Merck: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding.
OffLabel Disclosure:
Cyclosporine is an immunosuppressant. This trial assesses its application in hodgkin lymphoma
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal